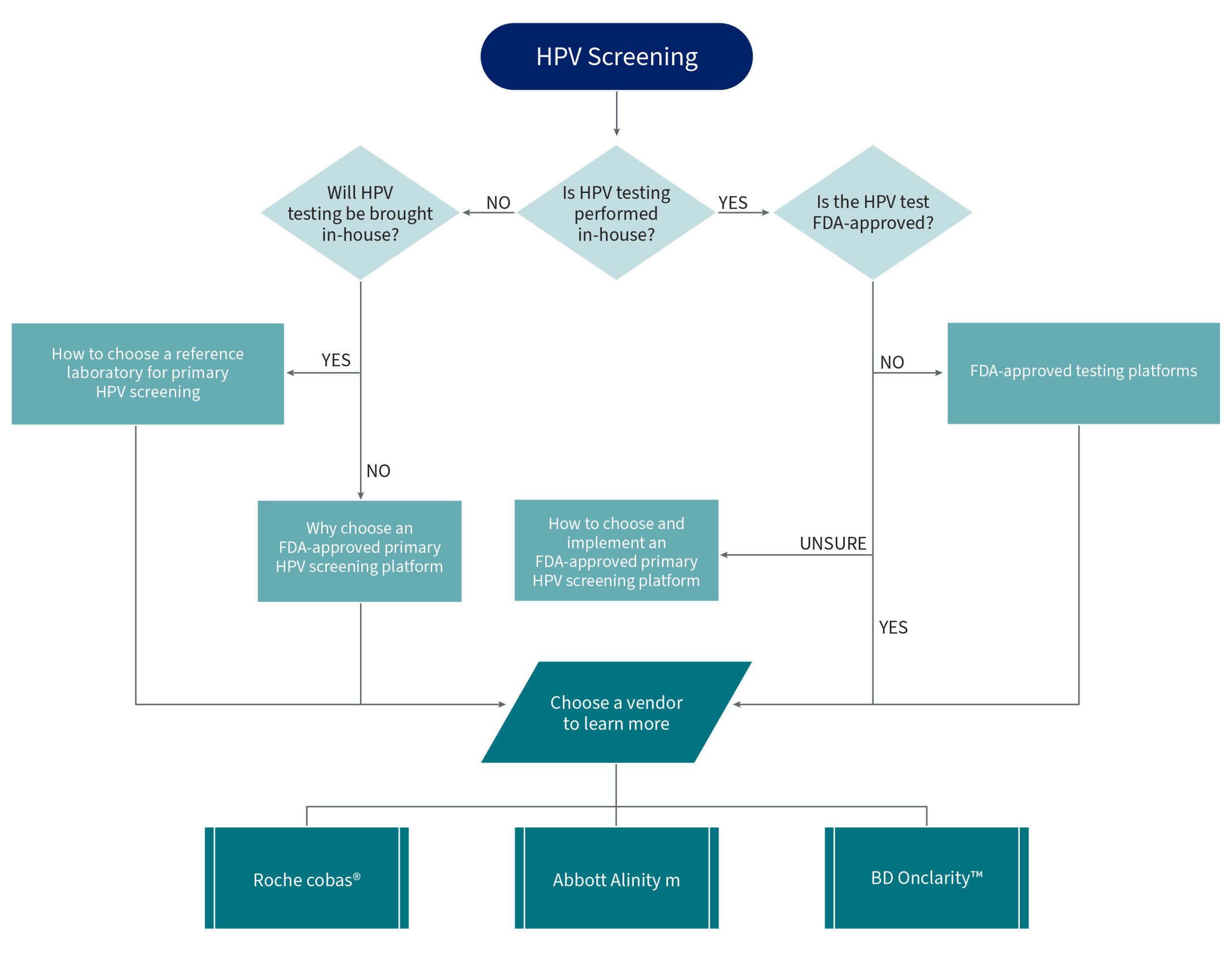
Primary HPV Screening Flowcharts
Implementation of Primary HPV Screening for Cervical Cancer – Laboratory Considerations
These flowcharts and the following resources have been developed and curated to support method evaluation for choosing a primary HPV testing platform.
HPV Screening
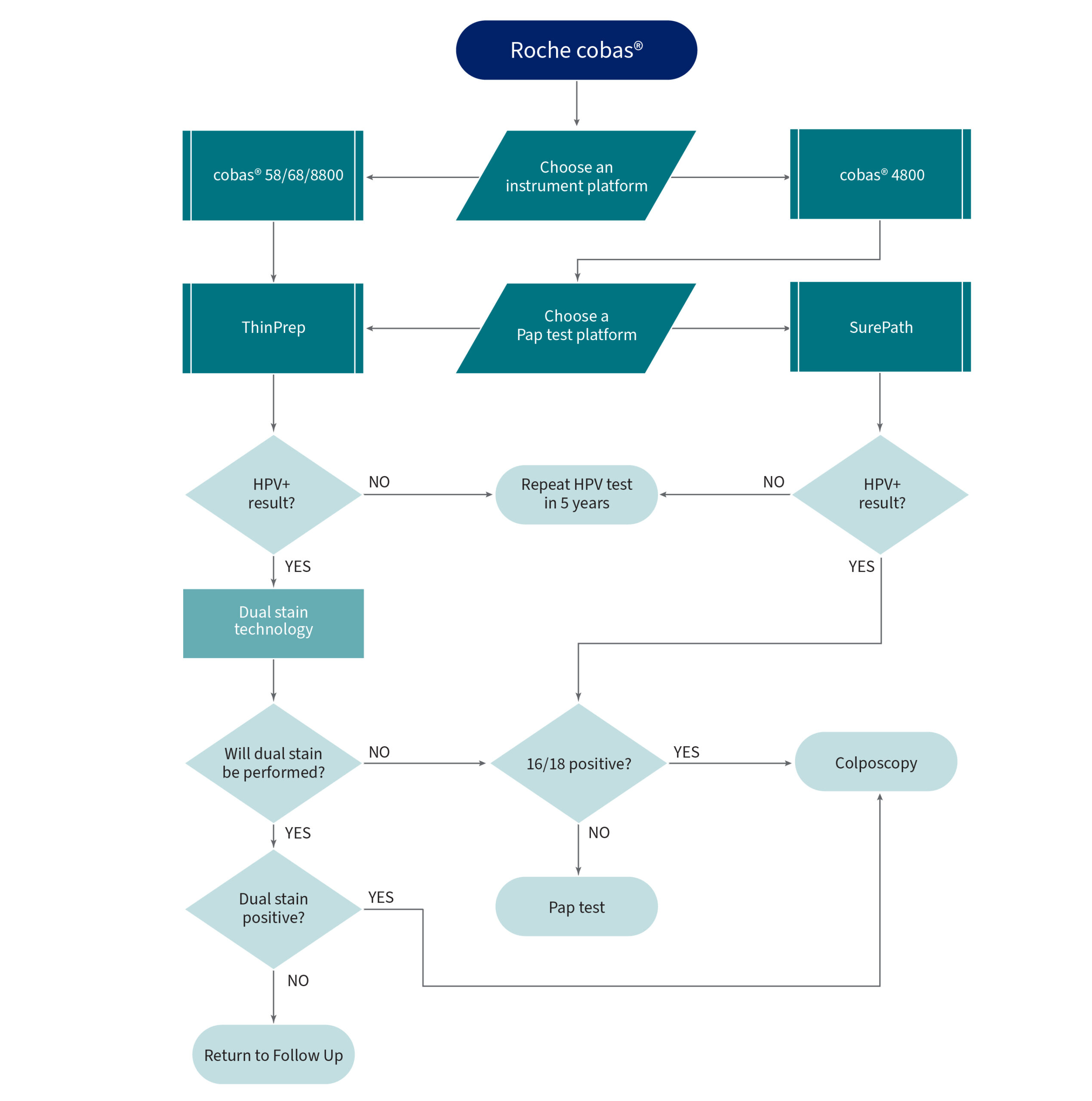
Roche cobas
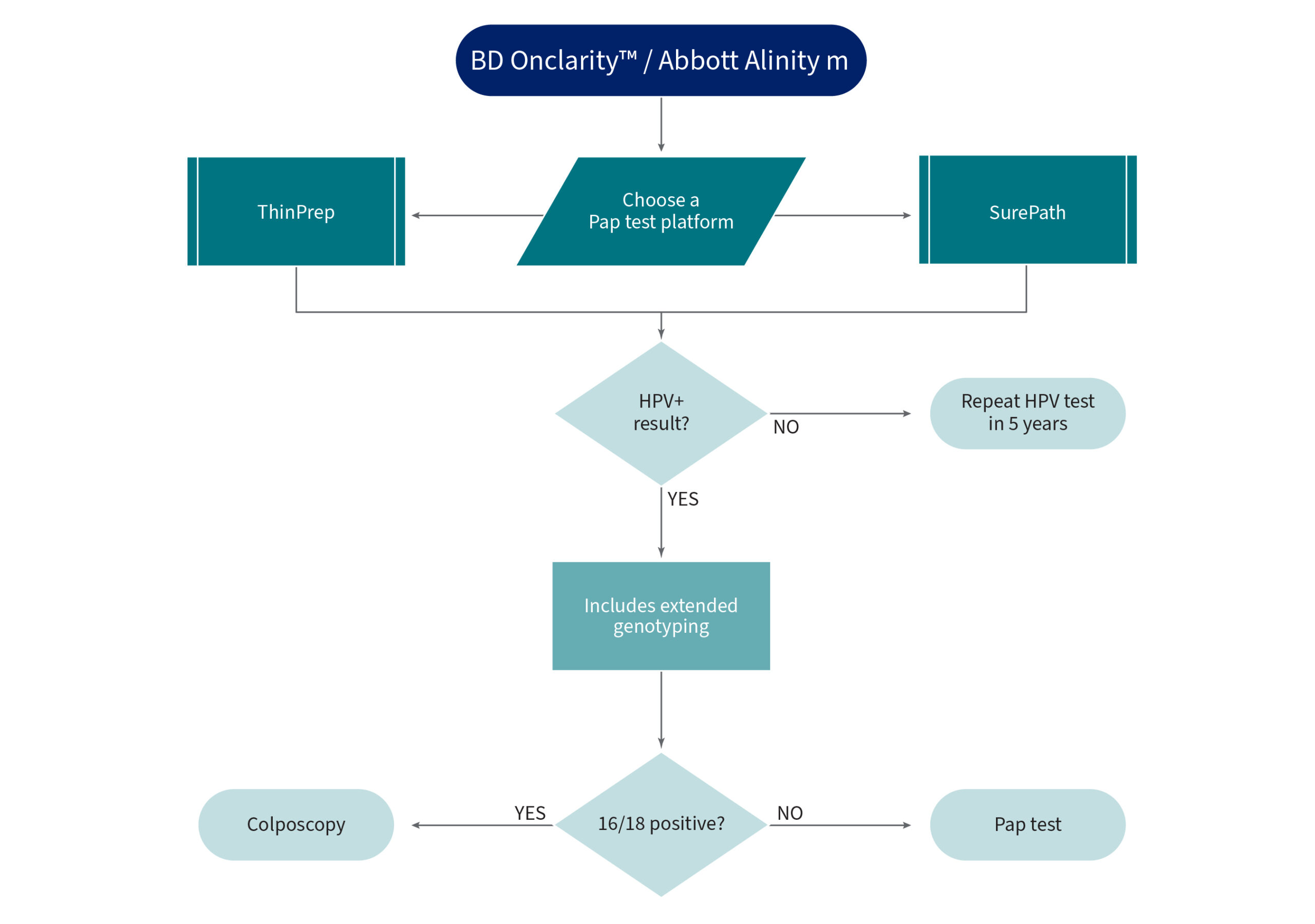
BD Onclarity / Abbott Alinity m
Resources
FDA-Approved Testing Platforms
- Salazar KL, Duhon DJ, Olsen R, Thrall M. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019 Sep-Oct;8(5):284-292. doi: 10.1016/j.jasc.2019.06.001. Epub 2019 Jun 13. PMID: 31320315. (Link)
- Alinity m Instrument: Abbott Molecular. Abbott Molecular. (Link)
- BD onclarity™ HPV assay. BD. (Link)
- Cobas® HPV. Roche Diagnostics. (Link)
How to Choose and Implement an FDA-Approved Primary HPV Screening Platform
- Cuschieri K, Fellner MD, Arroyo Mühr LS, Padalko E, Correa RM, Dillner J, Gultekin M, Picconi MA. Quality assurance in human papillomavirus testing for primary cervical screening. Int J Gynecol Cancer. 2023 May 1;33(5):802-811. doi: 10.1136/ijgc-2022-004197. PMID: 36914171; PMCID: PMC10176393. (Link)
- World Health Organization. Introducing and scaling up testing for human papillomavirus as part of a comprehensive programme for prevention and control of cervical cancer: a step-by-step guide. 2020;1-45. (Link)
- Clinical and Laboratory Standards Institute. Molecular diagnostic methods for infectious diseases; approved guideline. 2nd CLSI document MM3-A2. Vol. 26, No. 8. Wayne, PA: CLSI; 2006. (Can only be accessed by members.)
- Clinical and Laboratory Standards Institute. Verification and validation of qualitative nucleic acid tests; approved guideline. CLSI document MM17-A. Vol. 28, No. 9. Wayne, PA: CLSI; 2008. (Can only be accessed by members.)
Dual Stain Technology
- Khan R. Evolution of cervical cancer diagnostics: Dual-stain cytology. Roche Diagnostics. July 10, 2023. (Link)
- Gustafson LW, Tranberg M, Christensen PN, et al. Clinical utility of p16/Ki67 dual-stain cytology for detection of cervical intraepithelial neoplasia grade two or worse in women with a transformation zone type 3: A cross-sectional study. BJOG. 2023;130(2):202-209. doi:10.1111/1471-0528.17248 (Link)
- Stoler MH, Baker E, Boyle S, et al. Approaches to triage optimization in HPV primary screening: Extended genotyping and p16/Ki-67 dual-stained cytology-Retrospective insights from ATHENA. Int J Cancer. 2020;146(9):2599-2607. doi:10.1002/ijc.32669 (Link)
- Wright TC, Stoler MH, Ranger‐Moore J, et al. Clinical validation of P16/KI‐67 dual‐stained cytology triage of hpv‐positive women: Results from the impact trial. International Journal of Cancer. 2021;150(3):461-471. doi:10.1002/ijc.33812 (Link)
HPV Genotyping
- Stoler MH, Wright TC, Jr., Parvu V, Yanson K, Cooper CK, Andrews JA. Detection of high-grade cervical neoplasia using extended genotyping: Performance data from the longitudinal phase of the Onclarity trial. Gynecol Oncol. 2023;170:143-152. doi:10.1016/j.ygyno.2023.01.004 (Link)
- Stoler MH, Parvu V, Yanson K, Andrews J, Vaughan L. Risk stratification of HPV-positive results using extended genotyping and cytology: Data from the baseline phase of the Onclarity trial. Gynecol Oncol. 2023;174:68-75. doi:10.1016/j.ygyno.2023.01.004 (Link)
- Stoler MH, Baker E, Boyle S, et al. Approaches to triage optimization in HPV primary screening: Extended genotyping and p16/Ki-67 dual-stained cytology-Retrospective insights from ATHENA. Int J Cancer 2020;146(9):2599-2607. doi:10.1002/ijc.32669 (Link)
- Bottari F, Iacobone AD, Passerini R, et al. Human papillomavirus genotyping compared with a qualitative high-risk human papillomavirus test after treatment of high-grade cervical intraepithelial neoplasia: a systematic review. Obstet Gynecol. 2019;134(3):452-462. doi:10.1097/AOG.0000000000003409 (Link)
How to Choose a Reference Laboratory for Primary HPV Screening
- Qualifying, selecting, and evaluating a referral laboratory. 3rd ed. CLSI guideline QMS05. Wayne, PA: Clinical and Laboratory Standards Institute; 2020. (Link) (Can only be accessed by members.)
- Salazar KL, Duhon DJ, Olsen R, Thrall M. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019 Sep-Oct;8(5):284-292. doi: 10.1016/j.jasc.2019.06.001. Epub 2019 Jun 13. PMID: 31320315. (Link)
Why Choose An FDA-Approved Primary HPV Screening Platform
- WHO recommends DNA testing as a first-choice screening method for cervical cancer prevention. World Health Organization. September 11, 2021. (Link)
- Perkins RB, Wentzensen N, Guido RS, Schiffman M. Cervical cancer screening: a review. JAMA. 2023;330(6):547–558. doi:10.1001/jama.2023.13174. (Link)
- Stoler MH, Castle PE, Solomon D, Schiffman M. The expanded use of HPV testing in gynecologic practice per ASCCP-guided management requires the use of well-validated assays. American Journal of Clinical Pathology. 2007;127(3):335-337. doi:10.1309/rnf3c01jkadqclkp (Link)




